Invention Summary:
Peripheral Nerve Injuries (PNI) arising from physical trauma, cancers, or other nervous system pathologies are associated with higher costs and socioeconomic impact globally. Currently available technologies to remediate nerve injury are often unsuccessful in restoring nerve functions to its original capacity and cost of managing PNIs is on the rise. The autograft used in nerve graft surgery has 50% success rate with only 25-50% of pre-injury function is regained. Cadaveric allograft or even the artificial biopolymer conduit used lacks cells and trophic factors and may cause adverse condition if degraded.
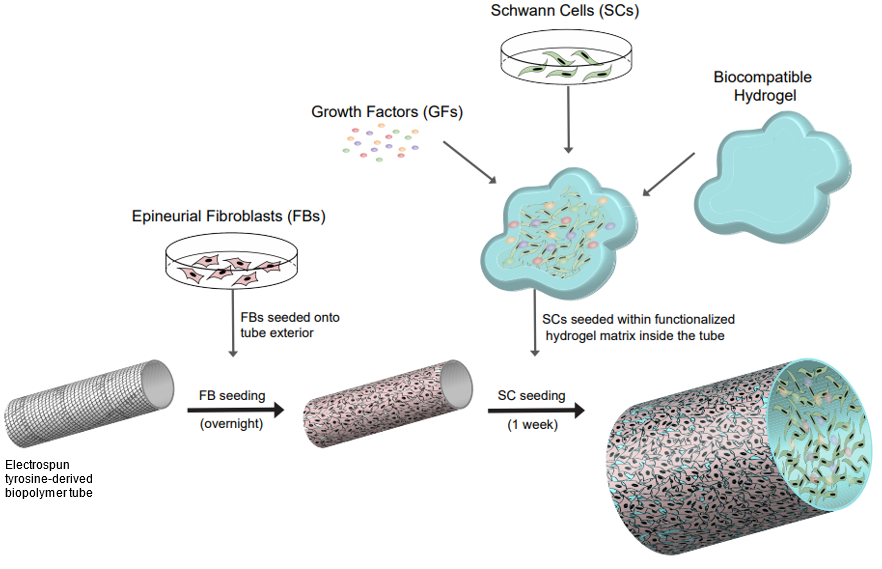
Rutgers researchers have invented a method to create a cellularized nerve regeneration conduit to treat peripheral nerve injuries. It is composed of a multilayer electrospun biodegradable polymer conduit, preferably tyrosine-based, in conjunction with an enclosed hydrogel-peptide matrix as the scaffold containing 3D-cultured autologous cells. The construct consists of epineural fibroblast cells on the outside surface and 3D-cultured Schwann cells with functionalized hydrogel in the lumen. It can promote neurite regrowth and myelination of injured neuronal axons. The graft mimics architecture of the nerve cord of the patient with high integrity.
Advantages:
- Avoid use of alternative healthy nerve
- High integrity nerve architecture with using patient‐derived Schwann cells and epineural fibroblasts
- Tyrosine-derived biopolymer with milder byproducts
- Improve clinical outcomes, reduce future costs to patients, insurers, and healthcare systems
Market Applications:
- Personalized nerve conduits/ implant, potentially more effective and with lower rejection rate
- Model to study peripheral nerve injuries
- Pathologic use for diabetic neuropathy
Intellectual Property & Development Status:
Patent pending. Available for licensing and/or research collaboration.