 Polymer-Alkyne Conjugates using UV sensitive monomer with linker
Polymer-Alkyne Conjugates using UV sensitive monomer with linker
Invention Summary:
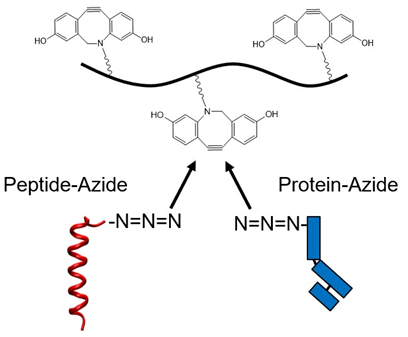
Biorthogonal “click” chemistry allows for the controlled introduction of multiple bioactive groups in a single molecule. Strain-promoted alkyne-azide cycloaddition (SPAAC) is an attractive click reaction in bioconjugate chemistry since it does not require the presence of toxic catalysts. A variety of compounds have been developed for efficient bioconjugation to azides; however, the intolerance of developed functional groups to radical polymerization reactions necessitates multi-step post-polymerization modifications and limits the number of polymer scaffolds that can be easily synthesized and tested.
Rutgers researchers have synthesized a monomer that allows for controlled derivatization of a polymer with bioactive molecules. The developed UV-sensitive monomer is compatible with a wide range of monomer families and can be incorporated onto the polymer backbone via oxygen-tolerant PETRAFT polymerization and can be subsequently utilized for conjugating the polymer to other macromolecules via SPAAC click chemistry.
Market Applications:
- Site-directed drug delivery
- Therapeutic treatments
- Bioengineering
Advantages:
- Monomer can be easily modified to change drug formulation.
- Eliminates use of multiple reagents in post functionalization of polymers
- Increases the efficiency of post functionalization.
Intellectual Property & Development Status:
Provisional patent application filed, patent pending. Available for licensing and/or search collaboration. For any business development and other collaborative partnerships contact marketingbd@research.rutgers.edu